SARS-CoV-2 Antigen Rapid Test Kit (CE marked)
Description
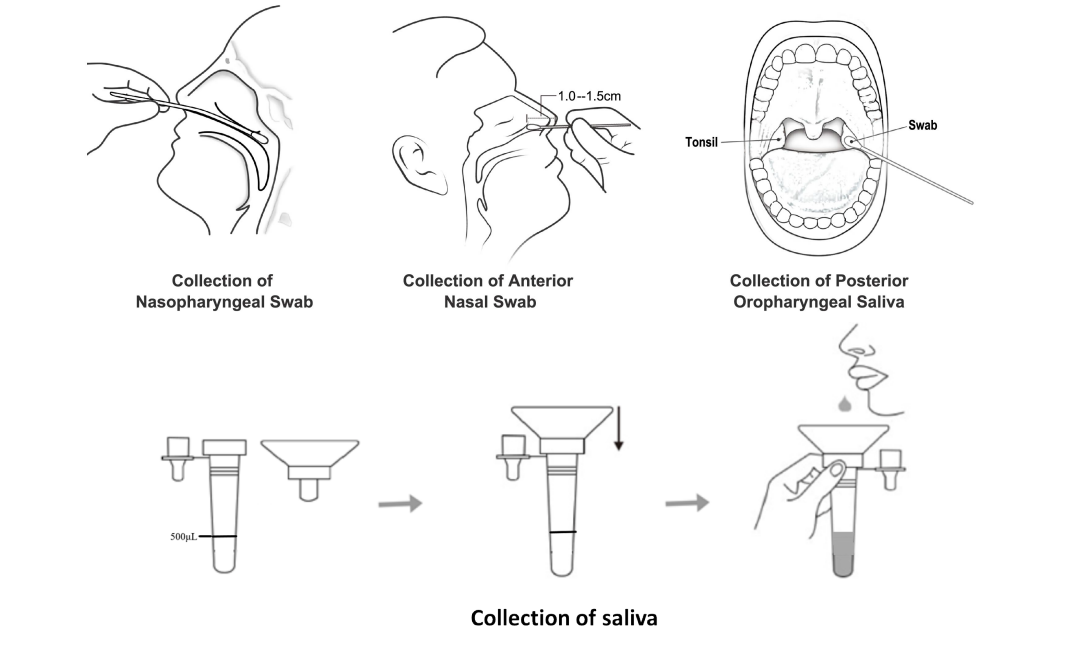
This kit is used for rapid in vitro qualitative detection of nucleocapsid protein (N protein) from SARS-CoV-2 antigen in human nasopharyngeal swabs, anterior nasal swab, or oropharyngeal swab samples. It can only be used as a supplementary detection index for suspected cases with negative nucleic acid detection in SARS-CoV-2, or used together with nucleic acid detection, and cannot be used as the only basis for the diagnosis and exclusion of pneumonia infected in SARS -CoV-2. The result of the testing is only for clinical reference, and it is suggested that the patient’s condition should be comprehensively analyzed in combination with clinical manifestations and other laboratory testing. The positive testing result needs further confirmation.
This kit is not only suitable for medical professionals to detect the N protein antigen of SARS-CoV-2, but also for layman self-testing.
Sample types:
- nasopharyngeal swab
- anterior nasal swab
- oropharyngeal swab
- saliva
Advantages and Features:
High sensitivity 98.51%; and high specificity 99.91% for early screening
Display results within 15 minutes
Qualifications: CE marked ; ISO 13485; More than 20 medical product registration certificates (NMPA and other countries)
Clinical Significance:
SARS-CoV-2 antigen detection kit can quickly detect positive cases when the viral load is high in acute infection period, and can be used for early shunt and rapid management of suspected population.
Packing Specifications:
1 test/kit, 5 tests/kit, 25 tests/ kit
Components:
Test card, Sample extraction solution, Sampler, IFU
Storage:
2℃~30℃, keep dry and away from light
Validity:
24 months